Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.
It is also used to oxidize the α-methylene group adjacent to a carbonyl group to give a 1,2-dicarbonyl compound. However selenium dioxide can perform several common types of oxidations, such as alcohols to ketones or aldehydes.
The oxidations of methylene groups using Selenium dioxide are referred to as Riley oxidations.
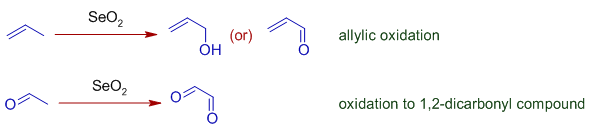
Note: SeO2 is sometimes referred to as selenium(IV) oxide.
Selenium oxide can also be used to oxidize alkynes in presence of acids. The internal alkynes are converted to 1,2-dicarbonyl compounds, whereas terminal alkynes are oxidized to glyoxylic acids.
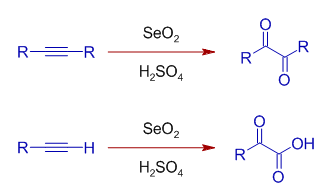
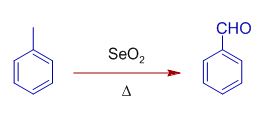
It also oxidizes benzylic methylene, CH2 group to C=O.

No comments:
Post a Comment