In previous post we learn about Metal metal bondong in low nuclear carbonyl clusters.
Now before moving on high nuclear carbonyl cluster we should talk about isolobal analogy.
PdF-Isolobal analogy|Organometallic chemistry
A SITE ABOUT CHEMISTRY, ALL CHEMISTRY TOPIC IN NOTES, CHEMISTRY NOTES IN HINDI AND ENGLISH, PROBLEMS OF CHEMISTRY AND THEIR SOLUTION FOR 11TH, 12TH, B.SC, M.SC AND NET/JRF STUDENTS
In previous post we learn about Metal metal bondong in low nuclear carbonyl clusters.
Now before moving on high nuclear carbonyl cluster we should talk about isolobal analogy.
PdF-Isolobal analogy|Organometallic chemistry
Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.
It is also used to oxidize the α-methylene group adjacent to a carbonyl group to give a 1,2-dicarbonyl compound. However selenium dioxide can perform several common types of oxidations, such as alcohols to ketones or aldehydes.
The oxidations of methylene groups using Selenium dioxide are referred to as Riley oxidations.
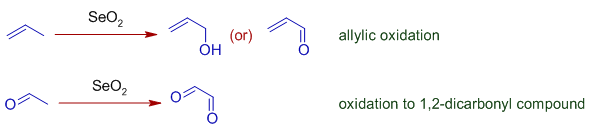
Note: SeO2 is sometimes referred to as selenium(IV) oxide.
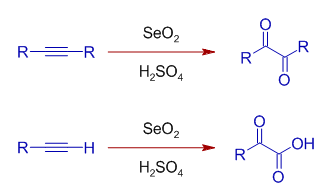
Selenium oxide can also be used to oxidize alkynes in presence of acids. The internal alkynes are converted to 1,2-dicarbonyl compounds, whereas terminal alkynes are oxidized to glyoxylic acids.
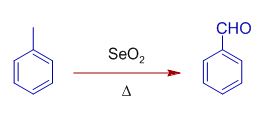
It also oxidizes benzylic methylene, CH2 group to C=O.
The Sarett oxidation is
an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively,
using
Unlike the similar Jones oxidation, the Sarett oxidation will not further oxidize primary alcohols to their carboxylic acid form, neither will it affect carbon-carbon double bonds.
Use of the original Sarett oxidation has become largely antiquated however, in favor of other modified oxidation techniques.
The unadulterated reaction is still occasionally used in teaching settings and in small scale laboratory research.
PDF-chromium based oxidizing reagent
In previous part we read about 18 electron rule in organometallic chemistry now here we will moving towards the cluster chemistry.
In organometallic there are two type of carbonyl clusters
LNCC AND HNCC
In LNCC we can find the structure by finding metal metal bond.
DOWNLOAD PDF-Metal metal bond in LNCC
CSIR NET PREVIOUS YEAR QUESTUONS-