A SITE ABOUT CHEMISTRY, ALL CHEMISTRY TOPIC IN NOTES, CHEMISTRY NOTES IN HINDI AND ENGLISH, PROBLEMS OF CHEMISTRY AND THEIR SOLUTION FOR 11TH, 12TH, B.SC, M.SC AND NET/JRF STUDENTS
Tuesday, December 7, 2021
Capping Principle||Organometallic Chemistry CSIR NET/GATE Inorganic Chemistry
Monday, August 9, 2021
Peroxide||Organic Reagent Series||CSIR NET/GATE/IIT JAM/M.SC CHEMISTRY
Peroxides are a group of compounds with the structure
R−O−O−R, where R = any element.
The O−O group in a peroxide is called the peroxide group or peroxo group.
The nomenclature is somewhat variable.
Sunday, July 11, 2021
Diastereoselctivity In Acyclic Alkene||Organic Chemistry From Clayden
Thursday, June 10, 2021
Bayer Villiger Oxidation||Reagent Chemistry
Baeyer-Villiger Oxidation
In Baeyer Villiger oxidation a carbonyl
Compound and an aromatic peracid react via insertion of the peroxo-O atom next to the C=O bond of the carbonyl compound.
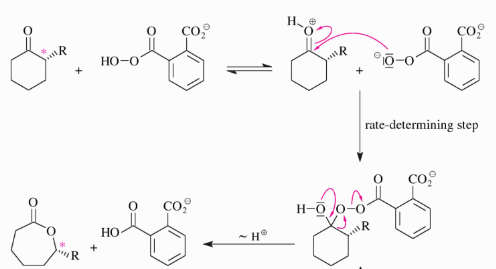
The Baeyer–Villiger Reaction is a commonly used oxidation reaction in organic synthesis is the conversion of carbonyl compounds into the corresponding esters or lactones
Thursday, June 3, 2021
m-CPBA REAGENT||oxidizing Reagent||CSIR NET CHEMISTRY
meta-Chloroperoxybenzoic acid (mCPBA or mCPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis.
mCPBA is often preferred to other peroxy acids because of its relative ease of handling.
mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.
OPPENAUER OXIDATION|CSIR NET REAGENT CHEMISRTY
Oppenauer Oxidation is the process of conversion of secondary alcohols to ketones by selective oxidation. This reaction is named after Rupert Viktor Oppenauer. Oxidation reaction takes place in the presence of [Al(i-Pro)3] in excess of acetone.
It is an aluminium alkoxide catalysed the oxidation of a secondary alcohol to the corresponding ketone. This is reverse of the Meerwein Ponndorf Verley reduction. It is a very good method to oxidize allylic alcohols to α, β- unsaturated ketones.
Friday, May 28, 2021
Isolobal Analogy||Organometallic Chèmistry
In previous post we learn about Metal metal bondong in low nuclear carbonyl clusters.
Now before moving on high nuclear carbonyl cluster we should talk about isolobal analogy.
PdF-Isolobal analogy|Organometallic chemistry
Sunday, May 23, 2021
SeO2-Selenium dioxide||Oxidizing reagent||CSIR NET ORGANIC CHEMISTRY
Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.
It is also used to oxidize the α-methylene group adjacent to a carbonyl group to give a 1,2-dicarbonyl compound. However selenium dioxide can perform several common types of oxidations, such as alcohols to ketones or aldehydes.
The oxidations of methylene groups using Selenium dioxide are referred to as Riley oxidations.

Note: SeO2 is sometimes referred to as selenium(IV) oxide.
Selenium oxide can also be used to oxidize alkynes in presence of acids. The internal alkynes are converted to 1,2-dicarbonyl compounds, whereas terminal alkynes are oxidized to glyoxylic acids.

It also oxidizes benzylic methylene, CH2 group to C=O.

Sunday, May 9, 2021
SARETT OXIDATION||chromium based oxidizing reagent
The Sarett oxidation is
an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively,
using
chromium trioxide and pyridine.
Unlike the similar Jones oxidation, the Sarett oxidation will not further oxidize primary alcohols to their carboxylic acid form, neither will it affect carbon-carbon double bonds.
Use of the original Sarett oxidation has become largely antiquated however, in favor of other modified oxidation techniques.
The unadulterated reaction is still occasionally used in teaching settings and in small scale laboratory research.
PDF-chromium based oxidizing reagent
Saturday, May 8, 2021
Metal Metal bond in organometallic LNCC ||CSIR NET ORGANOMETALLIC CHEMISTRY
In previous part we read about 18 electron rule in organometallic chemistry now here we will moving towards the cluster chemistry.
In organometallic there are two type of carbonyl clusters
LNCC AND HNCC
In LNCC we can find the structure by finding metal metal bond.
DOWNLOAD PDF-Metal metal bond in LNCC
CSIR NET PREVIOUS YEAR QUESTUONS-
Tuesday, April 13, 2021
18 Electron Rule-Organometallic Chemistry (CSIR NET/JRF,/GATE/IIT JAM)
Monday, February 22, 2021
PCC | PDC Reagent||CSIR NET CHEMISTRY||ORGANIC REAGENT||IIT JAM CHEMISTRY| GATE CHEMISTRY
Pyridinium dichromate is the pyridinium salt of dichromate that can be obtained by addition of pyridine to a solution of chromium trioxide in water.
The orange salt is commercially available and can by conveniently handled and stored; it is non-hygroscopic and soluble in many organic solvents. A similar salt is pyridinium chlorochromate (PCC), which shares the same properties. Both PDC and PCC can convert alcohols into aldehydes and ketones, especially in dichloromethane at room temperature. PDC is less acidic than PCC and is therefore more suitable for the oxidation of acid-sensitive substrates.
PDF-PCC &PDC REAGENT
Tuesday, February 2, 2021
Thermodynamics MCQ Part -2||RPSC COLLEGE PROFFESOR/GPSC/PPSC/1st Grade/SET/NEET/ CSIR NET CHEMISTRY
in this video we are covering MCQ of thermodynamics with tricks.
here we are covering RPSC college proffesor chemistry syllabus.
Here you will find questions related to 2nd law of thermodynamics, entropy, gibbs helmholtz equation, joule thomson effect.
All the questions are from previous year question paper of RPSC, NEET, SET,GPSC, CSIR NET EXAM.
this video will helpful for all the compation exams like CSIR NET, pgt chemistry, bhu chemistry, du entrance, phd entrance, NEET, IIT JAM, MH- SET, GSET and other exams.
Thursday, January 28, 2021
Thermodynamics MCQ Part -1||RPSC COLLEGE PROFFESOR/GPSC/PPSC/1st Grade/SET/NEET/ CSIR NET CHEMISTRY
in this video we are covering MCQ of thermodynamics with tricks.
here we are covering RPSC college proffesor chemistry syllabus.
All the questions are from previous year question paper of RPSC, NEET, SET,GPSC, CSIR NET EXAM.
this video will helpful for all the compation exams like CSIR NET, pgt chemistry, bhu chemistry, du entrance, phd entrance, NEET, IIT JAM, MH- SET, GSET and other exams.
Thermodynamics is a field of science that deals with the quantitative relationships between energy sources, and the study of heat and work interconversion. There are four rules of thermodynamics, and these are: zero law states that if two systems are in thermal equilibrium with a third system, then the first two systems are in thermal equilibrium. The first law states that, the total energy of an isolated system remains constant, it is a law of conservation of energy. The energy can neither be created nor destroyed, it can only be transferred from one form to another. The second law of thermodynamics states that, over time, the entropy of an isolated system that is not in equilibrium must rise and achieve the ultimate equilibrium value. Third law of thermodynamics states that the entropy of a system becomes constant as the temperature approaches absolute zero. The viability of a given transformation is determined by thermodynamics.
A thermodynamic process where no heat is exchanged with the surroundings is
- isothermal
- adiabatic
- isobaric
- isotropic
Answer: (b)



































